Hematopoietic cell self-renewal and differentiation
Using bone marrow derived stem and progenitor cells to understand blood development
Watch cell fate commitment unfold as a single bipotent Megakaryocytic-Erythroid Progenitor differentiates into megakaryocytes (green) and erythroid cells (red).
“What are the molecular mechanisms that regulate the development of healthy and cancerous blood cells?”
Using state of the art approaches including CRISPR, genetic screens, and timelapse microscopy, the Krause Lab works to identify the molecular mechanisms that regulate hematopoiesis. One major focus is determining how progenitors pick which differentiated cell type to become – this is cell fate commitment. Cell fate decisions are regulated by differential transcription and epigenetics and the cell cycle, in ways that are not yet understood. These processes affects the decision of the bipotent megakaryocyte-erythrocyte progenitor (MEP) to pick the megakaryocytic versus erythroid lineage.
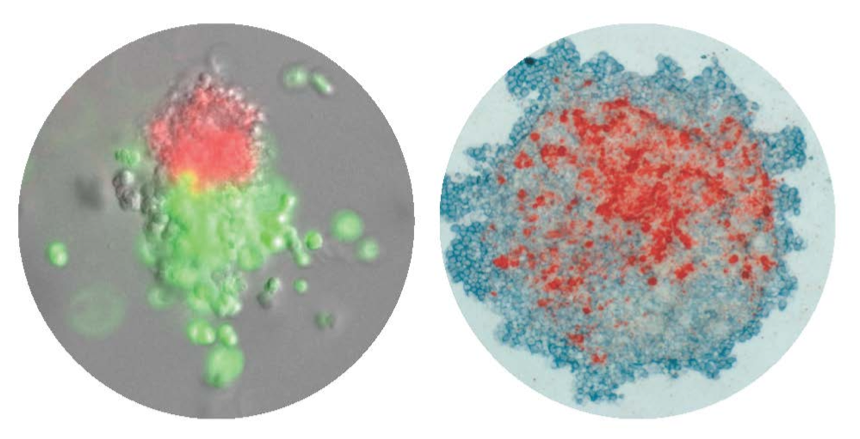
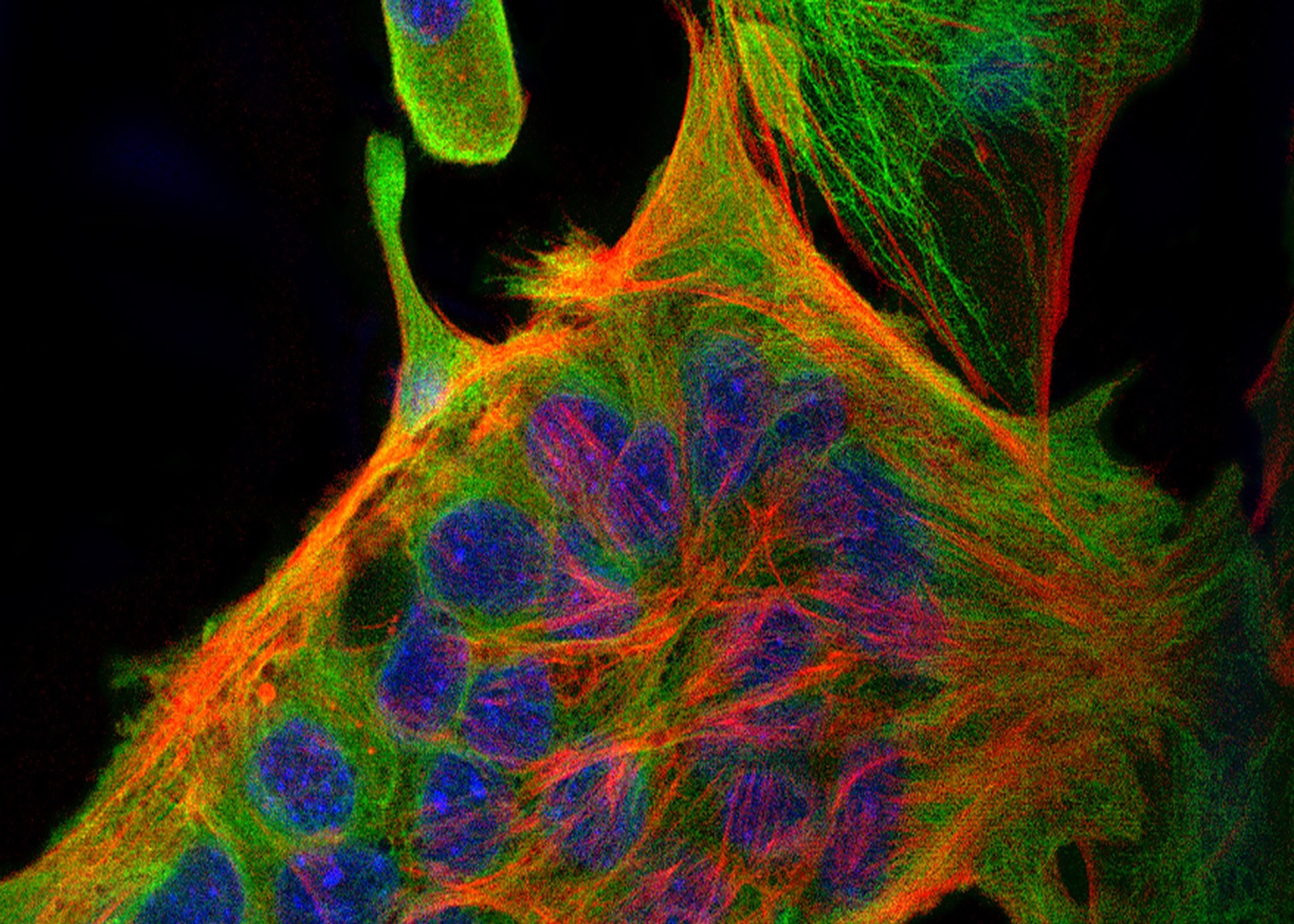
CFU-Mk/E: Colonies derived from a single cell that differentiated down the megakaryocytic and erythroid lineages. Left: Immunofluorescence, Right: Immunohistochemistry.
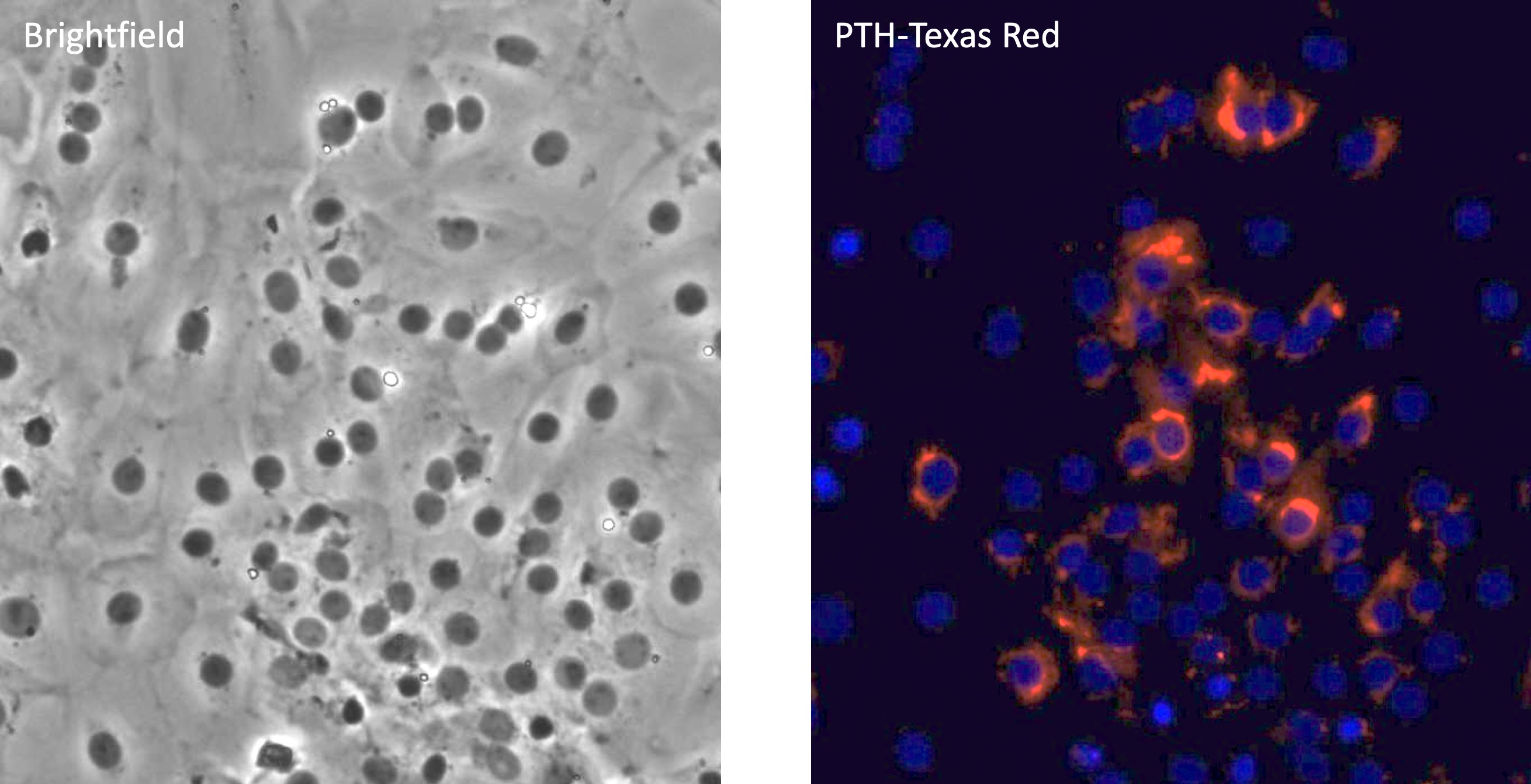
We have pioneered techniques for time-lapse imaging of the dynamics of differentiation in individual MEP (see video). The Krause lab has also developed immunohistochemistry and immunofluorescence methods that aid in high-throughput characterization of differentiation markers (see figure). In our ongoing research, we are applying cutting edge genomic approaches like single cell RNA-sequencing to finely study transcriptomic changes during the dynamic processes of hematopoiesis and leukemogenesis.